Page 19 - Curriculum Visions Dynamic Book
P. 19
Iron (Fe)
Element 26.A common metal element belonging to the transition metals in the Periodic Table.
Iron makes up about a twentieth of the Earth’s crust, being the second most abundant metal after aluminium. It is, however, far easier to separate it from its compounds than aluminium and has become the world’s most useful metal.
Iron is the most common magnetic element.
Iron is a steel-grey metal. It is quite reactive and readily corrodes to produce brown iron oxides, commonly known as rust.
Iron is also an important part of the human body. For example, it gives the red colour to blood.
Refined iron contains about 4% carbon.That makes it quite brittle.When most of the carbon is removed, it becomes easier to work. It is then called steel.
Discovery
Known since ancient times. Its smelting led to the Iron Age.
Small-scale production of iron by reacting iron oxide (iron ore) with powdered aluminium.A violent reaction takes place, reaching a temperature of over 2,000°C and producing molten iron and clouds of aluminium oxide smoke.This reaction, known as the thermit process, is used to weld railroad tracks together.
Key facts... Name: iron
Symbol: Fe
Atomic number: 26
Atomic weight: 55.84
Position in Periodic Table: transition metal, group
(8) (iron group); period 4
State at room temperature: solid
Colour: steel-grey
Density of solid: 7.86 g/cc
Melting point: 1,538°C
Boiling point: 2,760°C
Origin of name: from the Anglo-Saxon word iren.
The symbol Fe comes from the Latin word
ferrum, meaning iron.
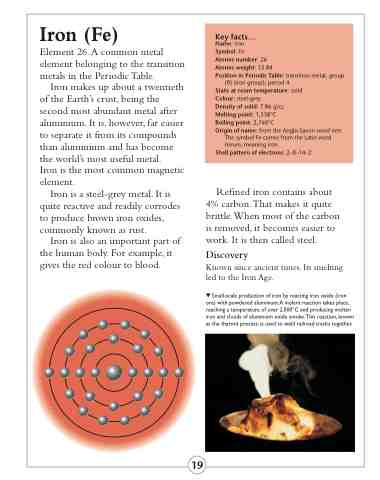
Shell pattern of electrons: 2–8–14–2
19