Page 18 - Curriculum Visions Dynamic Book
P. 18
Iridium (Ir)
Element 77. One of the platinum metals among the transition metals in the Periodic Table. It is a precious (very rare), brittle, silvery-white metal. It is one of the densest materials known. It is obtained as
a by-product of copper and nickel refining. Iridium is so unreactive that it cannot be dissolved even
by concentrated acids. Only concentrated molten salts can dissolve it.That makes it the most corrosion-resistant metal known.
Discovery
It was discovered in 1803 by the English chemist Smithson Tennant.
Technology
Biology
Iridium does not occur in living things. Because it is so unreactive, it is harmless.
Key facts...
Name: iridium
Symbol: Ir
Atomic number: 77
Atomic weight: 192.2
Position in Periodic Table: transition metal, group
(9) (cobalt group); period 6. Precious metal State at room temperature: solid
Colour: silvery-white
Density of solid: 22.5 g/cc
Melting point: 2,410°C
Boiling point: 4,527°C
Origin of name: from the Greek word iris, meaning
rainbow, because its compounds have a wide
variety of colours
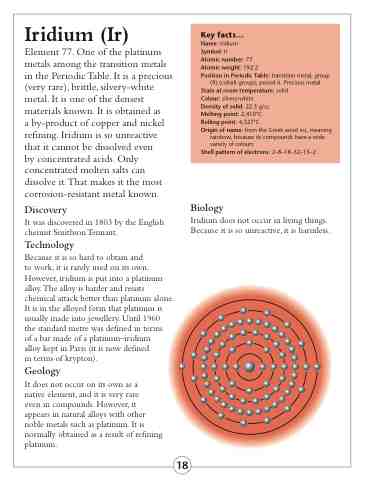
Shell pattern of electrons: 2–8–18–32–15–2
Because it is so hard to obtain and
to work, it is rarely used on its own. However, iridium is put into a platinum alloy. The alloy is harder and resists chemical attack better than platinum alone. It is in the alloyed form that platinum is usually made into jewellery. Until 1960
the standard metre was defined in terms
of a bar made of a platinum–iridium
alloy kept in Paris (it is now defined
in terms of krypton).
Geology
It does not occur on its own as a
native element, and it is very rare
even in compounds. However, it appears in natural alloys with other noble metals such as platinum. It is normally obtained as a result of refining platinum.
18