Page 13 - Curriculum Visions Dynamic Book. To close the book, close the tab.
P. 13
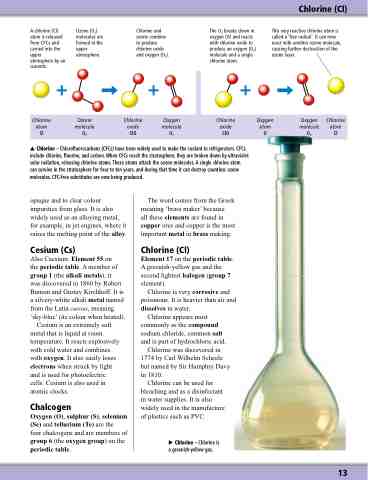
A chlorine (Cl) atom is released from CFCs and carried into the upper atmosphere by air currents.
Ozone (O3) molecules are formed in the upper atmosphere.
Chlorine and ozone combine to produce chlorine oxide and oxygen (O2).
The O2 breaks down in oxygen (O) and reacts with chlorine oxide to produce an oxygen (O2) molecule and a single chlorine atom.
This very reactive chlorine atom is called a ‘free radical’. It can now react with another ozone molecule, causing further destruction of the ozone layer.
Chlorine (Cl)
Chlorine Ozone atom molecule
Cl O3
Chlorine Oxygen oxide molecule
ClO O2
Chlorine Oxygen Oxygen Chlorine oxide atom molecule atom ClO O O2 Cl
Chlorine – Chlorofluorocarbons (CFCs) have been widely used to make the coolant in refrigerators. CFCs include chlorine, fluorine, and carbon. When CFCs reach the stratosphere, they are broken down by ultraviolet solar radiation, releasing chlorine atoms. These atoms attack the ozone molecules. A single chlorine atom can survive in the stratosphere for four to ten years, and during that time it can destroy countless ozone molecules. CFC-free substitutes are now being produced.
opaque and to clear colour impurities from glass. It is also widely used as an alloying metal, for example, in jet engines, where it raises the melting point of the alloy.
Cesium (Cs)
Also Caesium. Element 55 on
the periodic table. A member of group 1 (the alkali metals), it
was discovered in 1860 by Robert Bunsen and Gustav Kirchhoff. It is a silvery-white alkali metal named from the Latin caesius, meaning ‘sky-blue’ (its colour when heated).
Cesium is an extremely soft metal that is liquid at room temperature. It reacts explosively with cold water and combines with oxygen. It also easily loses electrons when struck by light and is used for photoelectric cells. Cesium is also used in atomic clocks.
Chalcogen
Oxygen (O), sulphur (S), selenium (Se) and tellurium (Te) are the
four chalcogens and are members of group 6 (the oxygen group) on the periodic table.
The word comes from the Greek meaning ‘brass maker’ because
all these elements are found in copper ores and copper is the most important metal in brass making.
Chlorine (Cl)
Element 17 on the periodic table. A greenish-yellow gas and the second lightest halogen (group 7 element).
Chlorine is very corrosive and poisonous. It is heavier than air and dissolves in water.
Chlorine appears most commonly as the compound sodium chloride, common salt and is part of hydrochloric acid.
Chlorine was discovered in 1774 by Carl Wilhelm Scheele but named by Sir Humphry Davy in 1810.
Chlorine can be used for bleaching and as a disinfectant in water supplies. It is also widely used in the manufacture of plastics such as PVC.
Chlorine – Chlorine is a greenish-yellow gas.
13