Page 30 - Curriculum Visions Dynamic Book
P. 30
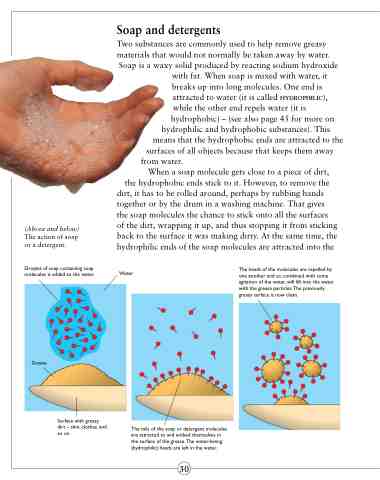
(Above and below)
The action of soap or a detergent.
Droplet of soap containing soap molecules is added to the water.
Soap and detergents
Two substances are commonly used to help remove greasy materials that would not normally be taken away by water.
Soap is a waxy solid produced by reacting sodium hydroxide with fat. When soap is mixed with water, it
breaks up into long molecules. One end is attracted to water (it is called hydrophilic), while the other end repels water (it is
hydrophobic) – (see also page 45 for more on hydrophilic and hydrophobic substances). This
means that the hydrophobic ends are attracted to the surfaces of all objects because that keeps them away
from water.
When a soap molecule gets close to a piece of dirt,
the hydrophobic ends stick to it. However, to remove the dirt, it has to be rolled around, perhaps by rubbing hands together or by the drum in a washing machine. That gives the soap molecules the chance to stick onto all the surfaces of the dirt, wrapping it up, and thus stopping it from sticking back to the surface it was making dirty. At the same time, the hydrophilic ends of the soap molecules are attracted into the
Water
The heads of the molecules are repelled by one another and so, combined with some agitation of the water, will lift into the water with the grease particles.The previously greasy surface is now clean.
Grease
Surface with greasy dirt – skin, clothes, and so on
The tails of the soap or detergent molecules are attracted to and embed themselves in the surface of the grease.The water-loving (hydrophilic) heads are left in the water.
30