Page 26 - Curriculum Visions Dynamic Book
P. 26
change. That is why most droplets on a table become flattened, and why when they fall through the air, they are not teardrop shaped (as popularly drawn) but become almost doughnut shaped.
Capillarity
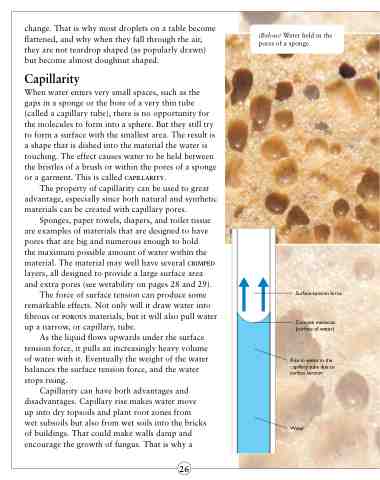
When water enters very small spaces, such as the gaps in a sponge or the bore of a very thin tube (called a capillary tube), there is no opportunity for the molecules to form into a sphere. But they still try to form a surface with the smallest area. The result is a shape that is dished into the material the water is touching. The effect causes water to be held between the bristles of a brush or within the pores of a sponge or a garment. This is called capillarity.
The property of capillarity can be used to great advantage, especially since both natural and synthetic materials can be created with capillary pores.
Sponges, paper towels, diapers, and toilet tissue are examples of materials that are designed to have pores that are big and numerous enough to hold
the maximum possible amount of water within the material. The material may well have several crimped layers, all designed to provide a large surface area and extra pores (see wetability on pages 28 and 29).
The force of surface tension can produce some remarkable effects. Not only will it draw water into fibrous or porous materials, but it will also pull water up a narrow, or capillary, tube.
As the liquid flows upwards under the surface tension force, it pulls an increasingly heavy volume of water with it. Eventually the weight of the water balances the surface tension force, and the water stops rising.
Capillarity can have both advantages and disadvantages. Capillary rise makes water move up into dry topsoils and plant root zones from
wet subsoils but also from wet soils into the bricks of buildings. That could make walls damp and encourage the growth of fungus. That is why a
(Below) Water held in the pores of a sponge.
Surface-tension force
Concave meniscus (surface of water)
Rise in water in the capillary tube due to surface tension
Water
26