Page 14 - Curriculum Visions Dynamic Book
P. 14
See Vol. 1: Plastics to find out more about plastics and polymerisation.
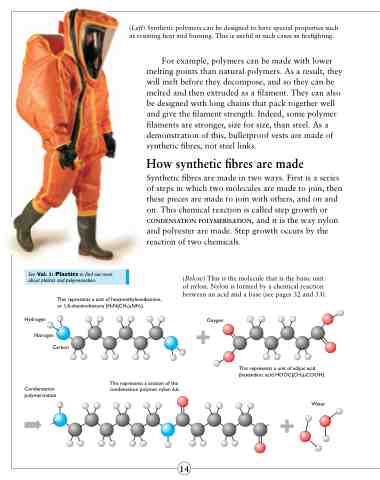
(Below) This is the molecule that is the basic unit of nylon. Nylon is formed by a chemical reaction between an acid and a base (see pages 32 and 33).
Hydrogen
Nitrogen Carbon
Condensation polymerisation
Oxygen
This represents a unit of hexamethylenediamine, or 1,6-diaminohexane (H2N(CH2)6NH2).
(Left) Synthetic polymers can be designed to have special properties such as resisting heat and burning. This is useful in such cases as firefighting.
For example, polymers can be made with lower melting points than natural polymers. As a result, they will melt before they decompose, and so they can be melted and then extruded as a filament. They can also be designed with long chains that pack together well and give the filament strength. Indeed, some polymer filaments are stronger, size for size, than steel. As a demonstration of this, bulletproof vests are made of synthetic fibres, not steel links.
How synthetic fibres are made
Synthetic fibres are made in two ways. First is a series of steps in which two molecules are made to join, then these pieces are made to join with others, and on and on. This chemical reaction is called step growth or condensation polymerisation, and it is the way nylon and polyester are made. Step growth occurs by the reaction of two chemicals.
This represents a section of the condensation polymer nylon 6,6.
This represents a unit of adipic acid (hexandioic acid HOOC(CH2)4COOH).
Water
14