Page 35 - Curriculum Visions Dynamic Book
P. 35
base: a compound that may be soapy to the touch and that can react with an acid in water to form a salt and water.
salts: compounds, often involving a metal, that are the reaction products of acids and bases. (Note “salt” is also the common word for sodium chloride, common salt or table salt.)
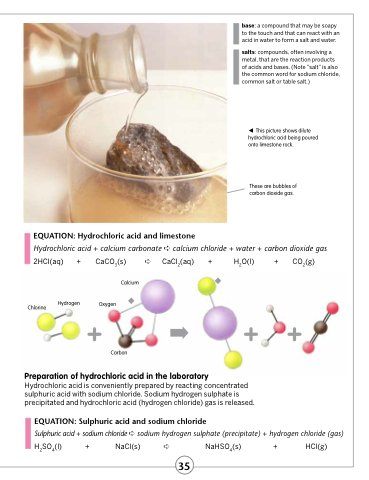
This picture shows dilute hydrochloric acid being poured onto limestone rock.
These are bubbles of carbon dioxide gas.
EQUATION: Hydrochloric acid and limestone
Hydrochloric acid + calcium carbonate ➪ calcium chloride + water + carbon dioxide gas
2HCl(aq) +
CaCO3(s) ➪ Calcium
CaCl2(aq)
+ H2O(l) + CO2(g)
◆
Chlorine
Hydrogen
Oxygen
◆
+➡++ Carbon
Preparation of hydrochloric acid in the laboratory
Hydrochloric acid is conveniently prepared by reacting concentrated sulphuric acid with sodium chloride. Sodium hydrogen sulphate is precipitated and hydrochloric acid (hydrogen chloride) gas is released.
EQUATION: Sulphuric acid and sodium chloride
Sulphuric acid + sodium chloride ➪ sodium hydrogen sulphate (precipitate) + hydrogen chloride (gas)
H2SO4(l) + NaCl(s) ➪
NaHSO4(s) + HCl(g)
35
35