Page 14 - Curriculum Visions Dynamic Book
P. 14
Chlorine as a bleaching agent
A bleach is a substance that removes stains from materials. It also has a disinfectant action.
There are two kinds of bleach: those that oxidise and those that reduce substances. Chlorine-based bleaches are oxidising agents.
Domestic bleach was traditionally made by passing chlorine gas over dry calcium oxide, producing calcium chlorate. When put in water this substance formed domestic bleach.
Powdered calcium chlorate, which can be added to water, and liquid solutions of sodium chlorate are both sold as domestic bleach (see page 24). In either case the compounds produced are quick and cheap to make, which accounts for the low price of most domestic bleaches.
Bleaches containing chlorine are powerful and are suited only to some fabrics, such as cotton, linen, and synthetic fibres. They cannot be used with wool, silk and some other materials, so in general chlorine-based bleaches are not used in detergent powders.
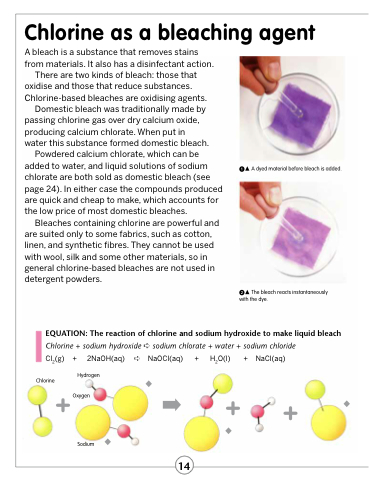
A dyed material before bleach is added.
EQUATION: The reaction of chlorine and sodium hydroxide to make liquid bleach
Chlorine + sodium hydroxide ➪ sodium chlorate + water + sodium chloride Cl2(g) + 2NaOH(aq) ➪ NaOCl(aq) + H2O(l) + NaCl(aq)
Hydrogen + Oxygen
The bleach reacts instantaneously with the dye.
Chlorine
◆
Sodium
◆
➡++ ◆
◆
14
14