Page 7 - Curriculum Visions Dynamic Book
P. 7
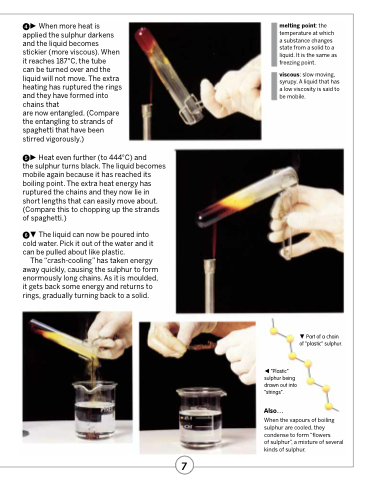
When more heat is applied the sulphur darkens and the liquid becomes stickier (more viscous). When it reaches 187°C, the tube
can be turned over and the liquid will not move. The extra heating has ruptured the rings and they have formed into chains that
are now entangled. (Compare the entangling to strands of spaghetti that have been stirred vigorously.)
Heat even further (to 444°C) and
the sulphur turns black. The liquid becomes mobile again because it has reached its boiling point. The extra heat energy has ruptured the chains and they now lie in short lengths that can easily move about. (Compare this to chopping up the strands of spaghetti.)
The liquid can now be poured into cold water. Pick it out of the water and it can be pulled about like plastic.
The “crash-cooling” has taken energy away quickly, causing the sulphur to form enormously long chains. As it is moulded, it gets back some energy and returns to rings, gradually turning back to a solid.
melting point: the temperature at which a substance changes state from a solid to a liquid. It is the same as freezing point.
viscous: slow moving, syrupy. A liquid that has a low viscosity is said to be mobile.
Part of a chain of “plastic” sulphur.
“Plastic” sulphur being drawn out into “strings”.
Also...
When the vapours of boiling sulphur are cooled, they condense to form “flowers
of sulphur”, a mixture of several kinds of sulphur.
7
7