Page 36 - Curriculum Visions Dynamic Book
P. 36
Reactions with copper sulphate
Copper sulphate is widely used in laboratory demonstrations. The colour makes the reactions easier to see. Here are some typical reactions.
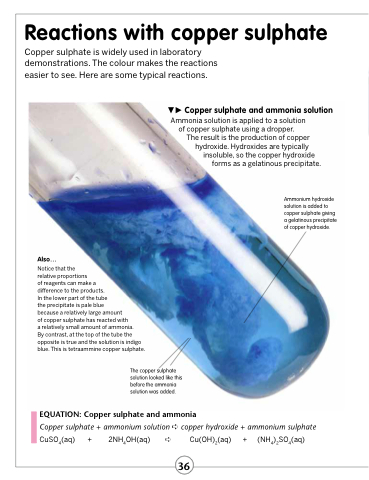
Copper sulphate and ammonia solution Ammonia solution is applied to a solution
of copper sulphate using a dropper.
The result is the production of copper hydroxide. Hydroxides are typically
insoluble, so the copper hydroxide forms as a gelatinous precipitate.
Ammonium hydroxide solution is added to copper sulphate giving a gelatinous precipitate of copper hydroxide.
Also...
Notice that the
relative proportions
of reagents can make a
difference to the products.
In the lower part of the tube
the precipitate is pale blue
because a relatively large amount
of copper sulphate has reacted with
a relatively small amount of ammonia.
By contrast, at the top of the tube the opposite is true and the solution is indigo blue. This is tetraammine copper sulphate.
The copper sulphate solution looked like this before the ammonia solution was added.
EQUATION: Copper sulphate and ammonia
Copper sulphate + ammonium solution ➪ copper hydroxide + ammonium sulphate
CuSO4(aq) + 2NH4OH(aq) ➪
Cu(OH)2(aq) + (NH4)2SO4(aq)
36
36