Page 40 - Curriculum Visions Dynamic Book
P. 40
Internal combustion
One of the most widely used fuels is petrol or gasoline, a carbon-based liquid that evaporates easily to release fumes. If the temperature of the gasoline is raised to over 350°C, as for example, by bringing a lighted match near to it, then the fumes (vapour) evaporating from the liquid will react with the oxygen in the surrounding air, creating carbon dioxide and water vapour and at the same time giving out a lot of heat.
If the fuel is formed into a mist of tiny droplets (it is “atomised”), such as happens to fuel being sucked into the cylinder of an engine, the fuel will burn much faster (see page 38). When the mist is ignited with a spark from a spark plug, combustion occurs and the liquid droplets (where
the molecules are tightly packed together) are transformed into a gas where the molecules are widely spaced. The rapid expansion of the gas (a controlled explosion) forces the piston down the cylinder, driving the vehicle, and also forces the spent gases out through the exhaust system. This process is called internal combustion.
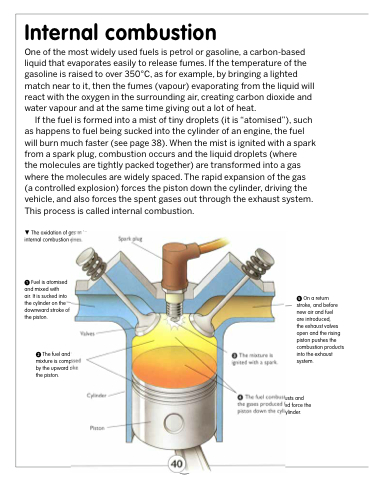
The oxidation of gases in internal combustion engines.
Fuel is atomised and mixed with
air. It is sucked into the cylinder on the downward stroke of the piston.
Valves
The fuel and air mixture is compressed by the upward stroke of the piston.
Cylinder
Piston
Spark plug
The mixture is ignited with a spark.
On a return stroke, and before new air and fuel
are introduced,
the exhaust valves open and the rising piston pushes the combustion products into the exhaust system.
The fuel combusts and the gases produced force the piston down the cylinder.
40 40