Page 29 - Curriculum Visions Dynamic Book
P. 29
How lead-acid batteries work
Lead-acid batteries are the most commonly used form of vehicle battery. To work as an electrical battery, each of the cells inside it must have two electrodes made of electrically conducting materials. These must be bathed in a liquid that can conduct electricity as well as help the battery store electricity. This material is called an electrolyte.
The electrodes, or plates, in lead-acid batteries are designed to have a large surface area. This allows the charge stored as chemical energy to be converted quickly into electrical energy. Half of the electrodes are made from a lead alloy covered in a paste of lead dioxide; the other half are made simply of lead alloy (known as “spongy” lead). All the plates are bathed in dilute sulphuric acid.
When a charge is drawn from the battery, a chemical reaction occurs between the plates and the sulphate ions of the electrolyte that changes the lead dioxide into lead sulphate.
When the battery is charged (perhaps from a vehicle generator), and a current passed through the battery, a chemical reaction occurs that turns the lead sulphate on the plates into lead and lead dioxide and returns the sulphate to the electrolyte, thus increasing the concentration of the acid.
When a load is applied to the battery, the reaction reverses,
the lead sulphate coatings re-form and the sulphuric acid is used up.
This process can be repeated many times, giving the secondary – battery a useful life of many years of constant service.
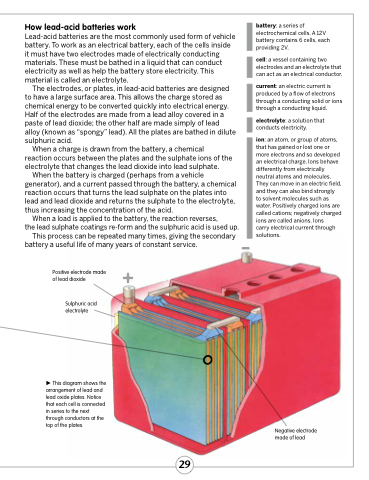
Positive electrode made + of lead dioxide
Sulphuric acid electrolyte
This diagram shows the arrangement of lead and lead oxide plates. Notice that each cell is connected in series to the next through conductors at the top of the plates.
battery: a series of electrochemical cells. A 12V battery contains 6 cells, each providing 2V.
cell: a vessel containing two electrodes and an electrolyte that can act as an electrical conductor.
current: an electric current is produced by a flow of electrons through a conducting solid or ions through a conducting liquid.
electrolyte: a solution that conducts electricity.
ion: an atom, or group of atoms, that has gained or lost one or more electrons and so developed an electrical charge. Ions behave differently from electrically neutral atoms and molecules. They can move in an electric field, and they can also bind strongly to solvent molecules such as water. Positively charged ions are called cations; negatively charged ions are called anions. Ions
carry electrical current through solutions.
Negative electrode made of lead
29