Page 21 - Curriculum Visions Dynamic Book
P. 21
decompose: to break down a substance (for example by heat or with the aid of a catalyst) into simpler components. In such a chemical reaction only one substance is involved.
electron: a tiny, negatively charged particle that is part of an atom. The flow of electrons through a solid material such as a wire produces an electric current.
infra-red radiation: a form
of light radiation where the wavelength of the waves is slightly longer than visible light. Most heat radiation
is in the infra-red band.
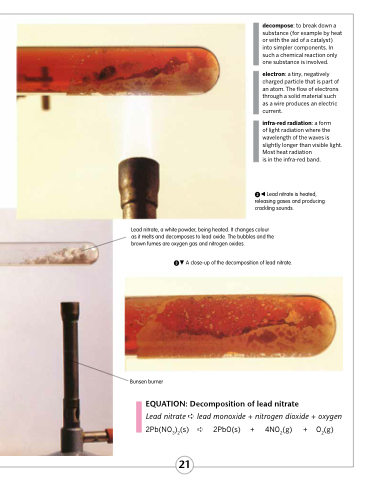
Lead nitrate is heated, releasing gases and producing crackling sounds.
Lead nitrate, a white powder, being heated. It changes colour as it melts and decomposes to lead oxide. The bubbles and the brown fumes are oxygen gas and nitrogen oxides.
A close-up of the decomposition of lead nitrate.
Bunsen burner
EQUATION: Decomposition of lead nitrate
Lead nitrate ➪ lead monoxide + nitrogen dioxide + oxygen 2Pb(NO3)2(s) ➪ 2PbO(s) + 4NO2(g) + O2(g)
21