Page 16 - Curriculum Visions Dynamic Book
P. 16
Carbon monoxide
Carbon monoxide is a reducing agent, that is
it takes oxygen from some materials it contacts. This is a useful reaction, and carbon monoxide
is used widely in industry, especially in the refining of metals from their ores. For example, the chemical reactions inside a blast furnace involve the reduction of iron ore to iron metal.
However, carbon monoxide is also produced when fuels are burned because the burning is not usually efficient enough to produce only carbon dioxide. Unless exhaust gases from burning fuels are allowed to disperse, this colourless, tasteless and odourless gas can build up and be fatal.
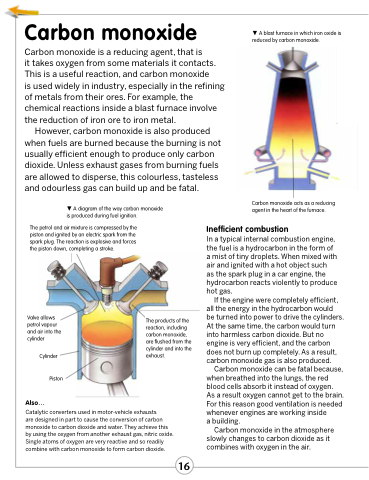
A diagram of the way carbon monoxide is produced during fuel ignition.
A blast furnace in which iron oxide is reduced by carbon monoxide.
The petrol and air mixture is compressed by the piston and ignited by an electric spark from the spark plug. The reaction is explosive and forces the piston down, completing a stroke.
Valve allows petrol vapour and air into the cylinder
Cylinder Piston
Also...
Catalytic converters used in motor-vehicle exhausts
are designed in part to cause the conversion of carbon monoxide to carbon dioxide and water. They achieve this by using the oxygen from another exhaust gas, nitric oxide. Single atoms of oxygen are very reactive and so readily combine with carbon monoxide to form carbon dioxide.
Carbon monoxide acts as a reducing agent in the heart of the furnace.
Inefficient combustion
In a typical internal combustion engine, the fuel is a hydrocarbon in the form of a mist of tiny droplets. When mixed with air and ignited with a hot object such
as the spark plug in a car engine, the hydrocarbon reacts violently to produce hot gas.
If the engine were completely efficient, all the energy in the hydrocarbon would
be turned into power to drive the cylinders. At the same time, the carbon would turn into harmless carbon dioxide. But no engine is very efficient, and the carbon does not burn up completely. As a result, carbon monoxide gas is also produced.
Carbon monoxide can be fatal because, when breathed into the lungs, the red blood cells absorb it instead of oxygen.
As a result oxygen cannot get to the brain. For this reason good ventilation is needed whenever engines are working inside
a building.
Carbon monoxide in the atmosphere
slowly changes to carbon dioxide as it combines with oxygen in the air.
The products of the reaction, including carbon monoxide, are flushed from the cylinder and into the exhaust.
16
16