Page 42 - Curriculum Visions Dynamic Book
P. 42
Gold alloys and gold plating
Gold is not only rare, it is a very soft metal. For many reasons it is sensible to alloy it with
other metals to improve its characteristics for use.
Gold is most often alloyed with copper and silver. This changes the strength and colour of the
alloy as well as making the gold go farther.
The resulting alloys are cheaper than pure gold.
“Green gold” is an alloy of gold, silver and
copper with silver in the greatest proportion. In “red gold” copper dominates the alloy, and an alloy of gold and nickel is called “white gold”.
Gold can be formed into alloys with mercury called amalgams; these are often used as dental fillings. Gold can also be plated on to objects to increase their resistance to corrosion.
The brooch shown here is a real orchid that has been coated in gold using electrolysis. The orchid was dipped in a resin, and the edges
of the petals painted with a metal paint so that it could be made into an electrical conductor. Then it was suspended in a bath of electrolyte. As an electric current flowed, the gold was deposited on to the metal-coated flower.
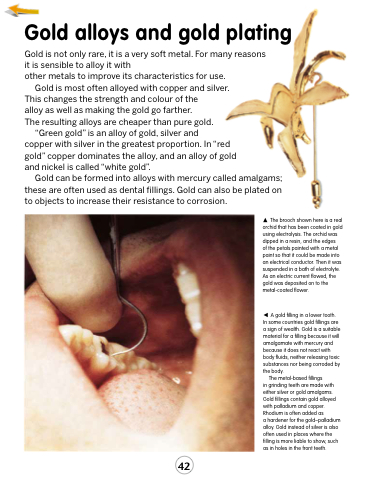
A gold filling in a lower tooth. In some countries gold fillings are a sign of wealth. Gold is a suitable material for a filling because it will amalgamate with mercury and because it does not react with body fluids, neither releasing toxic substances nor being corroded by the body.
The metal-based fillings
in grinding teeth are made with either silver or gold amalgams. Gold fillings contain gold alloyed with palladium and copper. Rhodium is often added as
a hardener for the gold–palladium alloy. Gold instead of silver is also often used in places where the filling is more liable to show, such as in holes in the front teeth.
42
42