Page 16 - Curriculum Visions Dynamic Book
P. 16
Copper as a conductor
All metals have the ability to transfer, or conduct, heat and electricity; however, copper is among the most efficient at conducting both.
An electric current is simply a flow of electrons. Metals can conduct electricity because they are made up of a “honeycomb” (known as
a lattice) of positively charged ions in a “sea” of electrons. In the case of copper, these electrons are not bound to any one copper ion but can move freely. This is what makes copper such a good conductor of electricity.
When heat is applied to copper, the atoms of the metal vibrate and pass energy across the honeycomb. Copper conducts heat better than most metals because of the arrangement of its atoms.
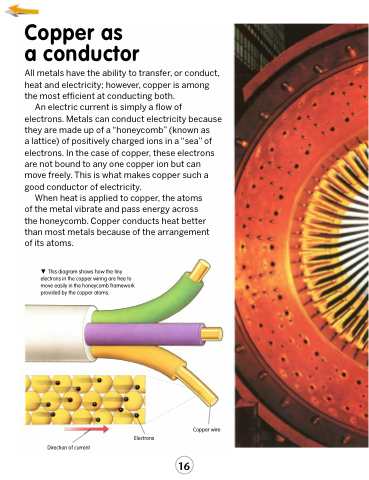
This diagram shows how the tiny electrons in the copper wiring are free to move easily in the honeycomb framework provided by the copper atoms.
Direction of current
Copper wire
Electrons
16
16