Page 8 - Curriculum Visions Dynamic Book
P. 8
Crystals containing calcium
The most common compound of calcium is limestone, calcium carbonate. This is often a dull grey rock, but occasionally, in small cavities in the rocks, it makes crystals, and the true brilliance of the mineral shows through. The crystalline form of calcium carbonate
is called calcite.
Calcite can be found all over the world. It often occurs
along with rare metals. In fact, because it is so common and has such a sparkling white colour, it is very
easy to spot. It has led prospectors to find such
metals as gold and tin, silver and copper.
Forms of calcite
There is a pure, completely transparent version of calcite. It is known as Iceland Spar because it is sometimes found
in cavities in solidified lava, of which Iceland is made. When you look into a piece of Iceland Spar, you actually
see double because it has the wonderful property of showing two images of anything you see through it.
More often, calcite forms sparkling
white crystals, which are sometimes found on the surface near hot springs. Geologists call this material travertine
(see pages 16 and 17). It was the most widely used building stone of ancient Rome.
Other calcium-rich crystals
Calcium compounds can also make crystals
known as “Blue John,” a corruption of the
French words bleu and jaune for blue and yellow. Geologists call it fluorspar (calcium fluoride) and it makes beautiful green, blue and yellow cubic crystals.
8 8
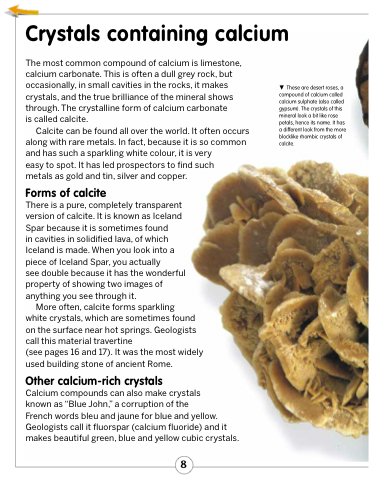
These are desert roses, a compound of calcium called calcium sulphate (also called gypsum). The crystals of this mineral look a bit like rose petals, hence its name. It has a different look from the more blocklike rhombic crystals of calcite.