Page 25 - Curriculum Visions Dynamic Book
P. 25
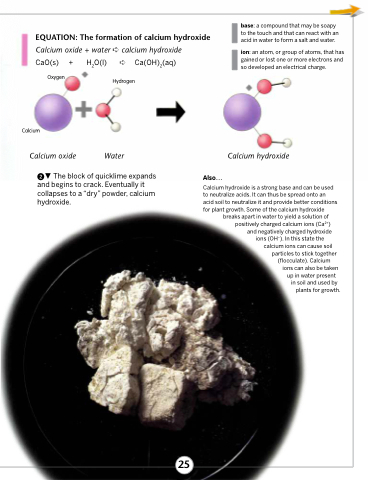
EQUATION: The formation of calcium hydroxide
base: a compound that may be soapy to the touch and that can react with an acid in water to form a salt and water.
ion: an atom, or group of atoms, that has gained or lost one or more electrons and so developed an electrical charge.
Calcium oxide + water ➪ calcium hydroxide
CaO(s) +
H2O(l) ➪ Ca(OH)2(aq) Hydrogen
Water
Oxygen
Calcium
Calcium oxide
Calcium hydroxide
Calcium hydroxide is a strong base and can be used to neutralize acids. It can thus be spread onto an acid soil to neutralize it and provide better conditions for plant growth. Some of the calcium hydroxide
breaks apart in water to yield a solution of positively charged calcium ions (Ca2+) and negatively charged hydroxide
ions (OH–). In this state the calcium ions can cause soil
particles to stick together (flocculate). Calcium
ions can also be taken up in water present
in soil and used by plants for growth.
The block of quicklime expands and begins to crack. Eventually it collapses to a “dry” powder, calcium hydroxide.
Also...
25 25