Page 9 - Curriculum Visions Dynamic Book
P. 9
The best place to look for crystals of sodium chloride is in the table salt
kept in every home. The crystals
can be seen with a magnifying
glass. The best place to look for crystals outside the home is in those places where saline solutions evaporate, such as coastal lagoons or desert lakes. The picture on the right
is of the surface of one of
the world’s best-known
salt deposits, on the floor
of Death Valley, California
(see also page 11). Notice
how the crystals have grown
up into tall square-sectioned protrusions. Thus they retain their cubic structure.
The most common compound of sodium is rock salt or halite. Rock salt is a glassy-looking (vitreous) material. Shown here is a crystalline mass of rock salt taken
from a salt mine. It is not easy to see individual crystals in crystalline material.
crystal: a substance that has grown freely so that it can develop external faces. Compare with crystalline, where the atoms are not free to form individual crystals and amorphous where the atoms are arranged irregularly.
crystalline: the organisation of atoms into a rigid “honeycomb-like” pattern without distinct crystal faces.
decompose: to break down a substance (for example by heat or with the aid
of a catalyst) into simpler components. In such a chemical reaction only one substance is involved.
mineral: a solid substance made of just one element or chemical compound. Calcite is a mineral because it consists only of calcium carbonate, halite is a mineral because it contains only sodium chloride.
translucent: almost transparent. vitreous: glass-like.
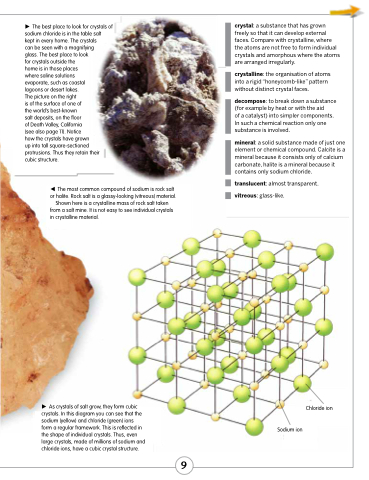
As crystals of salt grow, they form cubic crystals. In this diagram you can see that the sodium (yellow) and chloride (green) ions form a regular framework. This is reflected in the shape of individual crystals. Thus, even large crystals, made of millions of sodium and chloride ions, have a cubic crystal structure.
Chloride ion Sodium ion
9 9