Page 43 - Curriculum Visions Dynamic Book
P. 43
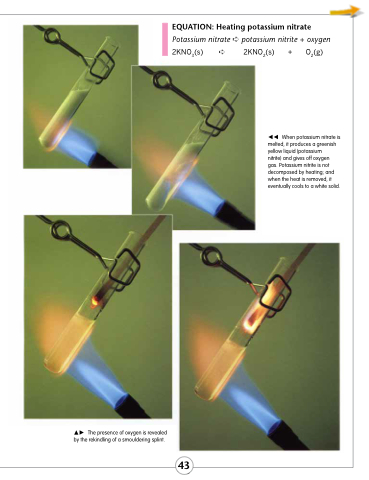
The presence of oxygen is revealed by the rekindling of a smouldering splint.
EQUATION: Heating potassium nitrate
Potassium nitrate ➪ potassium nitrite + oxygen
2KNO3(s) ➪
2KNO2(s) + O2(g)
When potassium nitrate is melted, it produces a greenish yellow liquid (potassium
nitrite) and gives off oxygen gas. Potassium nitrite is not decomposed by heating; and when the heat is removed, it eventually cools to a white solid.
43 43