Page 22 - Curriculum Visions Dynamic Book
P. 22
Salt solution as a conductor
A compound is a combination of two or more elements held together by small electrical charges. These charges work much like the electrical charge that is formed when you rub a balloon. The electrical charge created makes it “stick” to your clothes.
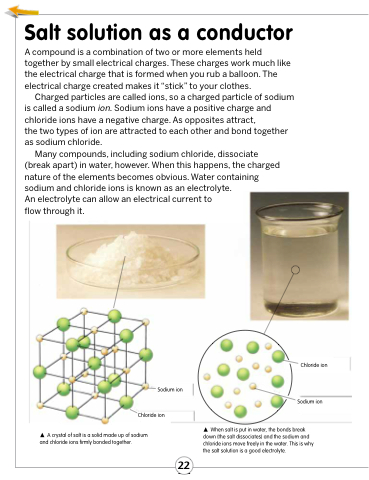
Charged particles are called ions, so a charged particle of sodium is called a sodium ion. Sodium ions have a positive charge and chloride ions have a negative charge. As opposites attract,
the two types of ion are attracted to each other and bond together as sodium chloride.
Many compounds, including sodium chloride, dissociate (break apart) in water, however. When this happens, the charged nature of the elements becomes obvious. Water containing sodium and chloride ions is known as an electrolyte.
An electrolyte can allow an electrical current to
flow through it.
Sodium ion Chloride ion
A crystal of salt is a solid made up of sodium and chloride ions firmly bonded together.
Chloride ion
Sodium ion
When salt is put in water, the bonds break down (the salt dissociates) and the sodium and chloride ions move freely in the water. This is why the salt solution is a good electrolyte.
22 22