Page 8 - Curriculum Visions Dynamic Book
P. 8
How hydrogen bonds
Hydrogen is found in many compounds.
The tiny hydrogen atoms bond to atoms of nitrogen, oxygen and fluorine in a very special way, like a special kind of glue. This is called hydrogen bonding.
Hydrogen bonding determines the way
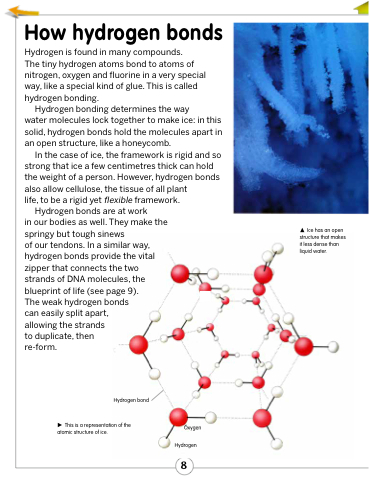
water molecules lock together to make ice: in this solid, hydrogen bonds hold the molecules apart in an open structure, like a honeycomb.
In the case of ice, the framework is rigid and so strong that ice a few centimetres thick can hold the weight of a person. However, hydrogen bonds also allow cellulose, the tissue of all plant
life, to be a rigid yet flexible framework. Hydrogen bonds are at work
in our bodies as well. They make the springy but tough sinews
of our tendons. In a similar way, hydrogen bonds provide the vital zipper that connects the two strands of DNA molecules, the blueprint of life (see page 9).
The weak hydrogen bonds can easily split apart, allowing the strands
to duplicate, then re-form.
Hydrogen bond
This is a representation of the atomic structure of ice.
Ice has an open structure that makes it less dense than liquid water.
Oxygen Hydrogen
8