Page 39 - Curriculum Visions Dynamic Book
P. 39
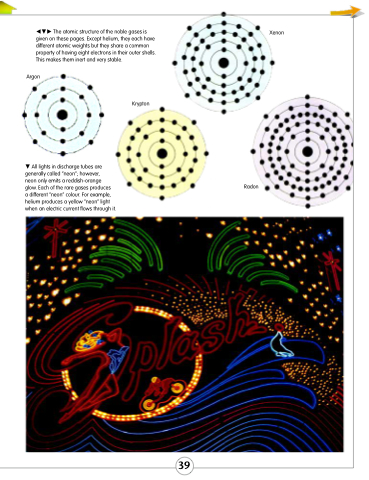
The atomic structure of the noble gases is given on these pages. Except helium, they each have different atomic weights but they share a common property of having eight electrons in their outer shells. This makes them inert and very stable.
Xenon
Argon
All lights in discharge tubes are generally called “neon”; however,
neon only emits a reddish-orange
glow. Each of the rare gases produces
a different “neon” colour. For example, helium produces a yellow “neon” light when an electric current flows through it.
Radon
Krypton
39