Page 49 - Curriculum Visions Dynamic Book
P. 49
Pb see Lead; Pd see Palladium Phosphorus (P)
Element 15. Phosphorus is a member of group 5 (the nitrogen group) in the Periodic Table.
There are several forms of phosphorus: colourless, white, red and black. Most commonly it is a white, soft, waxy solid
that is insoluble in water, that glows in the dark, is highly reactive in air and catches fire spontaneously. It turns into the red form when heated above 250°C or exposed to sunlight. Red phosphorus does not ignite spontaneously and so is less dangerous
than white phosphorus. Black phosphorus is rare and only formed at high temperatures.
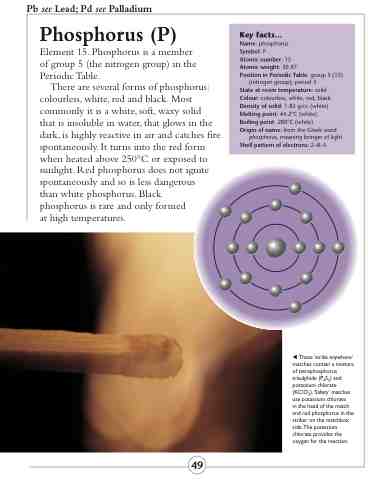
Key facts...
Name: phosphorus
Symbol: P
Atomic number: 15
Atomic weight: 30.97
Position in Periodic Table: group 5 (15)
(nitrogen group); period 3
State at room temperature: solid Colour: colourless, white, red, black Density of solid: 1.82 g/cc (white) Melting point: 44.2°C (white) Boiling point: 280°C (white)
Origin of name: from the Greek word
phosphoros, meaning bringer of light Shell pattern of electrons: 2–8–5
These ‘strike anywhere’ matches contain a mixture of tetraphosphorus trisulphide (P4S3) and potassium chlorate (KClO3).‘Safety’ matches use potassium chlorate
in the head of the match and red phosphorus in the striker on the matchbox side.The potassium chlorate provides the oxygen for the reaction.
49