Page 20 - Curriculum Visions Dynamic Book. To close the book, close the tab.
P. 20
Gold (Au)
Gold (Au)
Element 79 on the periodic table. A dense, heavy, shiny yellow precious metal and one of the transition metals.
Gold is soft and easily shaped (malleable) – it even makes thin sheets called gold leaf. It does not tarnish or corrode. It is found widely in pure native form, mainly in small pieces but occasionally
in large nuggets. That is why it was one of the first metals ever used by people.
Its low reactivity made it most easily turned into coins.
Gold does not stand up to continual handling, so to make it useful, it is alloyed with other metals. Most gold used in jewellery is an alloy with silver, copper,
or zinc. White gold is 70% silver. Gold alloys are measured in 24ths, units called carats – 24-carat gold is pure gold.
Gold is a good conductor of electricity and is plated over switch contacts and other places where
it is important that no corrosion occurs. Gold has also long been used for tooth fillings, partly because it doesn’t corrode and partly for its decorative effect.
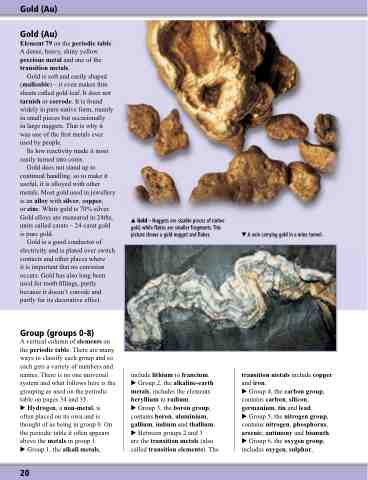
Gold – Nuggets are sizable pieces of native gold, while flakes are smaller fragments. This picture shows a gold nugget and flakes.
A vein carrying gold in a mine tunnel.
Group (groups 0-8)
A vertical column of elements on the periodic table. There are many ways to classify each group and so each gets a variety of numbers and names. There is no one universal system and what follows here is the grouping as used on the periodic table on pages 34 and 35.
Hydrogen, a non-metal, is often placed on its own and is thought of as being in group 0. On the periodic table it often appears above the metals in group 1.
Group 1, the alkali metals, 20
include lithium to francium.
Group 2, the alkaline-earth metals, includes the elements beryllium to radium.
Group 3, the boron group, contains boron, aluminium, gallium, indium and thallium. Between groups 2 and 3
are the transition metals (also called transition elements). The
transition metals include copper and iron.
Group 4, the carbon group, contains carbon, silicon, germanium, tin and lead.
Group 5, the nitrogen group, contains nitrogen, phosphorus, arsenic, antimony and bismuth. Group 6, the oxygen group, includes oxygen, sulphur,